HERBAL EXTRACTION UNIT:IntroductionSolvent extraction is usually used to recover a component from either a solid or liquid. The sample is contacted with a solvent that will dissolve the solutes of interest. Solvent extraction is of major commercial importance to the chemical and biochemical industries, as it is often the most efficient method of separation of valuable products from complex feedstock’s or reaction products. Some extraction techniques is involved in partition between two immiscible liquids, others involve in either continuous extractions or batch extractions. Because of environmental concerns, many common liquid/liquid processes have been modified to either utilize benign solvents, or move to more frugal processes such as solid phase extraction. The solvent can be a vapor, supercritical fluid or liquid and the sample can be a gas, liquid or solid. |
 |
SPRAY DRYER :Principles of Operation :Spray drying is a method of producing a dry powder from a liquid or slurry by rapidly drying with a hot gas. This is the preferred method of drying of many thermally-sensitive materials such as foods and pharmaceuticals. A consistent particle size distribution is a reason for spray drying some industrial products such as catalysts. Air is the heated drying media; however, if the liquid is a flammable solvent, such as ethanol or the product is oxygen sensitive nitrogen is used. |
 |
Unit Operations:Spray drying consists of the following unit operations :
*Pre-concentration of liquid |
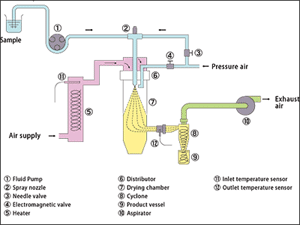 |
|
Spray drying applications:* Food: coffee, tea, eggs, cereal, spices, flavorings * Pharmaceutical: antibiotics, medical ingredients, additives * Industrial:paint pigments, ceramic materials, catalyst supports. |
Supercritical fluid extraction: |
Supercritical Fluid Extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes or on a larger scale to either strip unwanted material from a product (e.g. decaffeination) or collect a desired product (e.g. essential oils). Carbon dioxide (CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical CO2 are above the critical temperature of 31°C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. |
 |